I.c. Amino acids
Nitrogen-containing amino acids are essential for life and they are the building blocks of proteins. Amino acids, as ancient and ubiquitous molecules, have been co-opted by evolution for a variety of purposes in living systems. The importance in reading this section is limited to those who wants to visualize the structures.
1.c.i Amino acid Structure:
There are basically 20 standard amino acids having different structures in their side chains(R groups) . The common amino acids are known as a-amino acids because they have a primary amino group(-NH2) and a carboxylic acid group(-COOH) as substitutes of the a carbon atoms. Proline is an exception because it has a secondary amino group (-NH-), for uniformity it is also treated as alpha-amino acid.
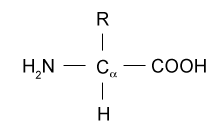
Fig 1.c.1. General structure of a- amino acid.
1.c.ii General properties:
The amino and carboxylic acid groups of amino acids readily ionize. At a pH(~7.4), the amino groups are protonated and the carboxyl acid groups are in their conjugate base(carboxylate) form, this shows that an amino acid that can act as an Acid and also a base. Amino acids can bear charged groups of opposite polarity, hence they are know as zwitterions or dipolar ions. The ionic property of the side chains influences the physical and chemical property of free amino acids and amino acids in proteins.
1.c.iii. Peptide bonds:
Elimination of water (condensation) can polarize amino acids to form long chains. The resulting CO-NH linkage, an amide linkage, is known as peptide bond. Polymers composed of two, three, a few(3 - 10), and many amino acid units are known, respectively, as dipeptides, tripeptides, oligopeptides, and polypeptides, commonly they are called "peptides".
II.c.iv. Classification:
There are basically three major classifications for amino acids (1) those with nonpolar R group, (2) those with uncharged polar R groups, and (3) those with charged polar R group. The table below shows us all 20 amino acids with their codes.
|
|
One-letter code
|
Three-letter-code
|
Name
|
|
1
|
A
|
Ala
|
Alanine
|
|
2
|
C
|
Cys
|
Cysteine
|
|
3
|
D
|
Asp
|
Aspartic Acid
|
|
4
|
E
|
Glu
|
Glutamic Acid
|
|
5
|
F
|
Phe
|
Phenylalanine
|
|
6
|
G
|
Gly
|
Glycine
|
|
7
|
H
|
His
|
Histidine
|
|
8
|
I
|
Ile
|
Isoleucine
|
|
9
|
K
|
Lys
|
Lysine
|
|
10
|
L
|
Leu
|
Leucine
|
|
11
|
M
|
Met
|
Methionine
|
|
12
|
N
|
Asn
|
Asparagine
|
|
13
|
P
|
Pro
|
Proline
|
|
14
|
Q
|
Gln
|
Glutamine
|
|
15
|
R
|
Arg
|
Arginine
|
|
16
|
S
|
Ser
|
Serine
|
|
17
|
T
|
Thr
|
Threonine
|
|
18
|
V
|
Val
|
Valine
|
|
19
|
W
|
Trp
|
Tryptophan
|
|
20
|
Y
|
Tyr
|
Tyrosine
|
Table 1.c.4.1: 20 Standard amino acids.
Non-polar amino acid side chains have a variety of shapes and sizes, there are basically nine acids under this classification. Glycine has the smallest possible side chain, an H atom. Alanine, valine, leucine, and isoleucine have aliphatic hydrocarbon side chains ranging in size from a methyl group for alanine to isomeric butyl groups for leucine and isoleucine. Methionine has a thiol ether side chain that resembles an n-butyl group in many of its physical properties(C and A have nearly equal electronegativities, and S is about the size of a methylene group). Proline has a cyclic pyrrolidine side group. Phenylalanine(with its phenyl moiety) and typtophan(with its indole group) conaint aromatic side groups, which are characterized by bulk as well as nonpolarity.
Uncharged Polar side chains have Hydroxyl, Amide, or Thiol Groups
There are six amino acids under this. Serine and threonine have hydroxylic R side chains of different sizes. Asparagine and glutamine have amide-bearing side chains of different sizes. Tyrosine has a phenolic group and is aromatic. Cystein is very unique among all 20 amino acid because it has a thiol group that form a disulfide bond with other Cystein through oxidation.
Charged Polar side chains, they are positively or negatively charged. Five amino acids contribute to this type. The side chains are positively charged; they are lysine, which has a butylammonium side chain, arginine, which has a guanidine group, and histidine, which bears an imidazolium moiety.
1.c.v. Nomenclature:
The three letter and single letter abbreviations are given table 2.c.4.1, most of them are taken from the first three letters and pronounced and written for eg. The symbol Glx in dicates Glu or Gln, care should be taken while writing these notations. The one-letter notations are used while comparing sequences of several similar proteins.
With this we end our discussion about amino acids and you don't have to worry about them until visualizing of a protein comes into picture.
During translation the genetic code in the form of RNA is translated into a protein sequence like in the fig. 1.c.1.

Fig. 2.c.1 synthesis of protein sequence from a mRNA
| 








